https://www.ucm.es/signalyeast/

Group photo. From left to right, Óscar Barbero-Oriz, Humberto Martín, María Molina, Víctor J. Cid, Graciela Alonso, Alejandro Fernández-Vega, Beatriz Lavilla-García, Teresa Fernández-Acero, Isabel Rodríguez-Escudero and Sara López-Montesino
Our laboratory is located in the heart of the Moncloa campus of the Complutense University. Around the common axis of cell signaling routes in eukaryotes, we work on different lines, some with a basic orientation focused on the study of yeast signaling routes Saccharomyces cerevisiae and others applied to the development of this cellular model for genetic and molecular studies of human pathologies.
Signaling routes are essential for all cells, from the most elementary prokaryotic systems to the most complex upper eukaryotes. They allow the detection and adaptation of physicochemical changes in the environment, as well as intercellular communication. Highly preserved throughout evolution, these routes respond to various stimuli to regulate crucial physiological processes for cell survival and proliferation control. From cloning and characterization three decades of the gene SLT2, which encodes the MAPK (mitogen activated kinase protein) responsible for the maintenance of cell wall integrity (CWI), the line of research directed by Teresa Fernández-Acero, Humberto Martín and María Molina, has focused on the study of the function of various components of this route, the mechanisms that control its activation and regulation, especially those related to phosphorylation/paragraph Interaction with other signaling routes.
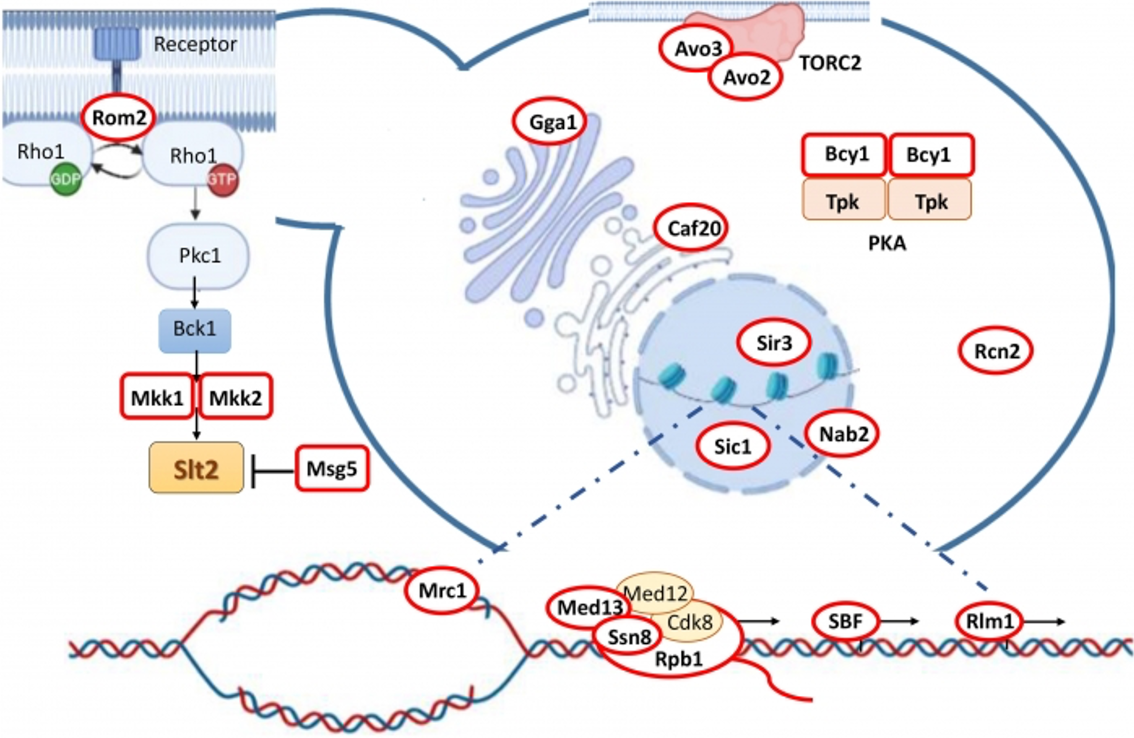
Figure 1. Scheme of the CWI route and SLT2 substrates (surrounded in red) in different cell locations (González-Rubio et al. 2022. J Fungi 8: 368).
On the other hand, knowledge about cell signaling in the homeostasis of yeast allows us to reprogram the system through the introduction of heterologists. In this context the group, in a line directed by the Drs. Isabel Rodríguez Escudero, Víctor J. CID and María Molina, we work on the elucidation of the function of virulence protein of intracellular pathogenic microorganisms through their expression in their expression in their expression S. cerevisiae and its interaction with procariotic functions, with emphasis on cell signaling. Throughout the group's history we have collaborated with other laboratories in the study of effectors of Legionella, Klebsiella, Brucella, Coxiella, Salmonella and Escherichia coli Enteropathogenic involved in the establishment of the intracellular niche and the evasion of the immune system.
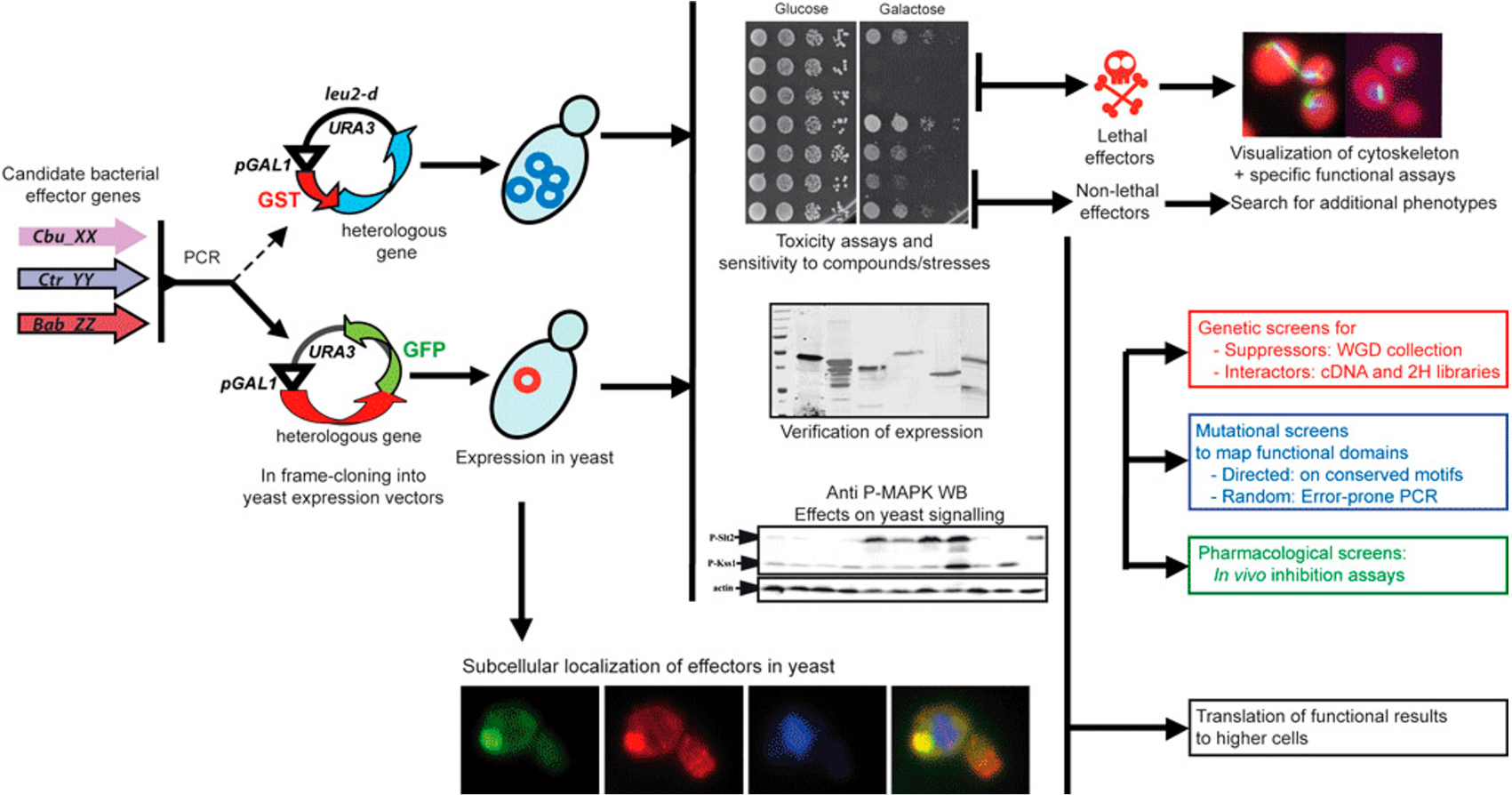
Figure 2. Work diagram for yeast studies of bacterial virulence of unknown function.
Another applied line of the laboratory, directed by the Drs. Teresa Fernández-Acero, Víctor J. Cid and María Molina focuses on applying synthetic biology strategies to reconstitute human cell signaling routes in the yeast chassis. In this context we have reconstituted in this model the human cancer pathway PI3K, which has allowed the development of functional tests on said oncogen, its effector, the AKT kinase protein and the Pten tumor suppressor. Currently, in collaboration with researchers from Harvard Medical School and other laboratories, we are working on the assembly of human signalosomes related to innate immunity, such as Myddosoma in the signage of TLR, inflameoma or the Cgas-Sting route.
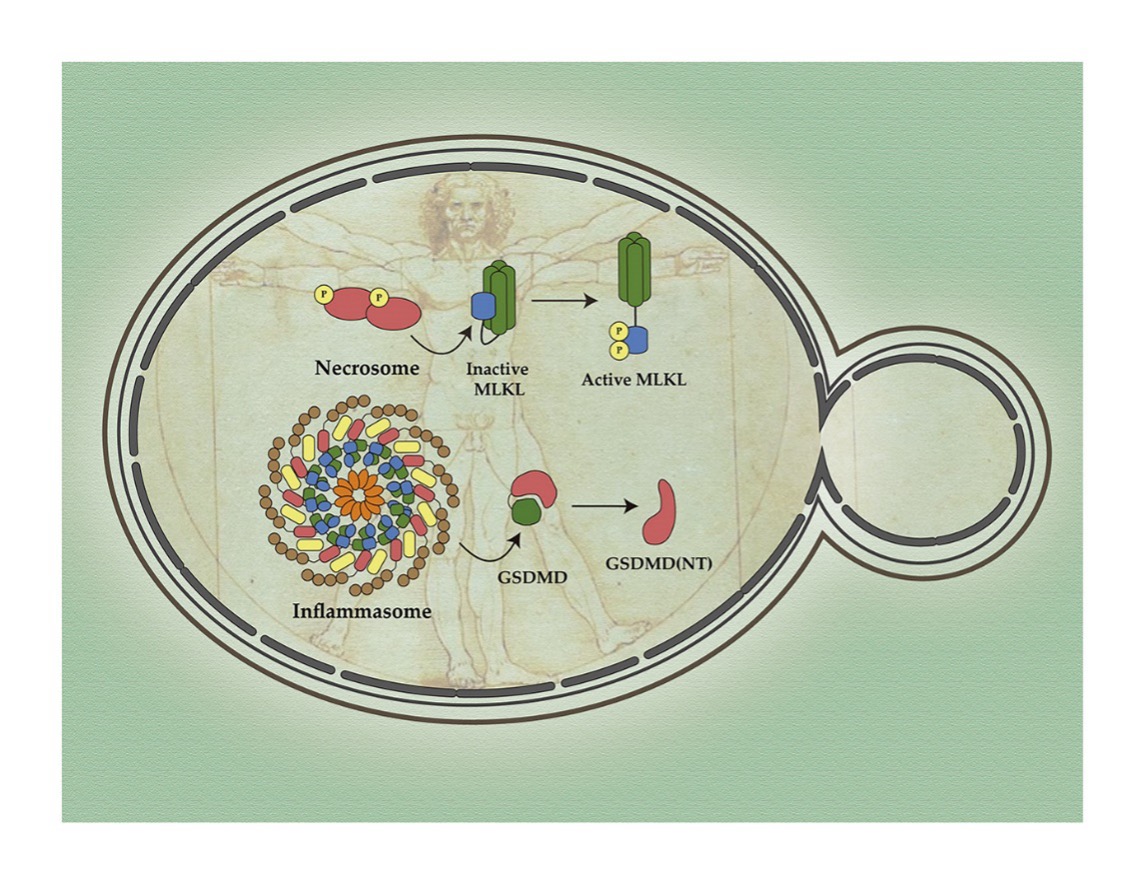
Figure 3. The assembly of innate immunity routes and cell death regulated in the yeast model by heterologist expression of its components allows the development of platforms for genetic and pharmacological studies (Barber -úriz et al. 2025. Biomolecules. 15:530)
Main articles of the group in the last 5 years:
Barber -úriz or, Valenti M, Molina M, Fernández-Acero T, Cid VJ. (2025). Modeling necropTotic and pyroptototic signaling in Saccharomyces cerevisiae. Biomolecules. 15(4):530. doi: 10.3390/biom15040530.
Valenti M, Molina M, Cid VJ. (2023). Human gasdermin D and MLKL disrupt mitochondria, endocytic traffic and TORC1 signalling in budding yeast. Open Biol.13: 220366. doi.org/10.1098/rsob.220366
González-Rubio G, Martín H, Molina M. (2023) The Mitogen-Activated Protein Kinase Slt2 Promotes Asymmetric Cell Cycle Arrest and Reduces TORC1-Sch9 Signaling in Yeast Lacking the Protein Phosphatase Ptc1. Microbiol Spectr. e0524922. doi: 10.1128/spectrum.05249-22.
Sá-Pessoa J, López-Montesino S, Przybyszewska K, Rodríguez-Escudero I, Marshall H, Ova A, Schroeder GN, Barabas P, Molina M, Curtis T, Cid VJ, Bengoechea JA. (2023). A trans-kingdom T6SS effector induces the fragmentation of the mitochondrial network and activates innate immune receptor NLRX1 to promote infection. Nat Commun. 14(1):871. doi: 10.1038/s41467-023-36629-3. PMID: 36797302.
Torices L, Mingo J, Rodríguez-Escudero I, Fernández-Acero T, Luna S, Nunes-Xavier CE, López JI, Mercadillo F, Currás M, Urioste M, Molina M, Cid VJ, Pulido R. (2022) Functional analysis of PTEN variants of unknown significance from PHTS patients unveils complex patterns of PTEN biological activity in disease. Eur J Hum Genet. doi: 10.1038/s41431-022-01265-w.
de Oya IG, Jiménez-Gutiérrez E, Gaillard H, Molina M, Martín H, Wellinger RE. (2022). Manganese Stress Tolerance Depends on Yap1 and Stress-Activated MAP Kinases. Int J Mol Sci. 23:15706. doi: 10.3390/ijms232415706.
Jiménez-Gutiérrez and, Fernandez-Azero T, Alonso-Rodríguez and, Molina M, Martin H. (2022) Neomy interferences by interference in Cew Phosphatidylisitoll-4 In j mol sci. ;23(19):11034 doi: 10,3390/Ijms2319111034
González-Rubio G, Sastre-Varagara L, Molina M, Martín H, Fernández-Acero T. (2022) Sublates of the Mapk SLT2: Shaping Yaest Cell Integraity. J Fungi (Basel). 8 (4): 368. DOI: 10.3390/JOF8040368.
Sellers-Moya Á, Nuévalos M, Molina M, Martín H. (2021). Clotrimazole-Induced Oxidative Stress Triggers Novel Yeast Pkc1-Independent Cell Wall Integrity MAPK Pathway Circuitry. J Fungi (Basel). 7(8):647. doi: 10.3390/jof7080647.
Coronas-Serna, JM, del Val E, Kagan JC, Molina M, Cid VJ (2021). Heterologous Expression and Assembly of Human TLR Signaling Components in Saccharomyces cerevisiae. Biomolecules, 11(11), 1737; doi.org/10.3390/biom11111737
Valenti M, Molina M, Cid VJ. (2021). Heterologous Expression and Auto-Activation of Human Pro-Inflammatory Caspase-1 in Saccharomyces cerevisiae and Comparison to Caspase-8. Front Immunol. 12:668602 doi: 10.3389/fimmu.2021.668602.
Luna s, tocares l, Mingo j, AMO L, RODRíguez-escuder I, Ruiz-Ibarlucea p, Erramuzpe A, Cortés JM, Tejada Mi, Molina M, Nunes-Xavier CE, López JJ, CID VJ, POLITIVE R. (2021). A Global Analysis of the Reconstitution of Pten Function by Translational Readthrough of Pten Pathogenic Premature Termination Codons. Hum mutat. 42(5):551-5 DOI: 10.1002/humu.2
González-Rubio G, Sellers-Moya Á, Martín H, Molina M. (2021). A walk-through MAPK structure and functionality with the 30-year-old yeast MAPK Slt2. Int Microbiol. doi: 10.1007/s10123-021-00183-z.
González-Rubio G, Sellers-Moya Á, Martín H, Molina M. (2021) Differential Role of Threonine and Tyrosine Phosphorylation in the Activation and Activity of the Yeast MAPK Slt2. Int J Mol Sci. 23;22(3):1110. doi: 10.3390/ijms22031110.PMID: 33498635
Imbert PRC, Imbert PRC, Rodriguez-Scuer I, Terradot (2020) Brucella abortus impact NAD metabolism. PLoS Pathogens. 16(4):e1007979. doi: 10.1371/journal.ppat.1007979. PMID: 32298382.
Storey D, McNally A, åstrand M, Sa-Pessoa Graca Santos J, Rodriguez-Escudero I, Elmore B, Palacios L, Marshall H, Habley L, Molina M, Cid VJ, Salminen Ta, Bengoechea Ja. (2020) Klebsiella pneumoniae type VI secretion system-mediated microbial competition is PhoPQ controlled and reactive oxygen species dependent. PLoS Pathog. 16(3):e1007969. doi: 10.1371/journal.ppat.1007969. PMID: 32191774.
Jiménez-Gutez-Gutiérrez and, happy-carrasco and, Alondo-Rodríguez and, Fernández-azier T, Molina M, Martin H. (2020). Rewiring The yeaast Cell Integrity (CWi) Pathway trough a symphic more feedback to the unveil unveils to the novel for the mapkk ssk2 in CWi Pathway's Action. Febs J. Doi: 10.1111/Febs.15288. pmid: 32150787
