
Group photo
Candida albicans is the most frequently isolated causative agent in fungal infections, but during the past two decades there has been a continuous increase in reported cases of mucosal and disseminated infections caused by Candida glabrata, Candida parapsilosis and Candida tropicalis. Worldwide it is considered C. glabrata as the second most prevalent causal agent of the genus Candida. Treatment of infections caused by C. glabrata is complicated because clinical isolates often show low susceptibility to the most common antifungal therapies. The establishment of infections C. glabrata it is mediated by the cell wall of the fungus that establishes primary pathogen-host interactions. For example, the cell wall contains adhesins that cause the adhesion of the fungus to biomedical surfaces and the formation of biofilms. Therefore, these adhesins play an important role in the infectious process and because similar proteins are absent in mammalian cells, they represent suitable candidates for the development of new antifungal drugs, antifungal vaccines,Candida effective and more precise and systematic methods to diagnose fungal infections.
We have described that the genome of C. glabrata contains an exceptionally large family of genes encoding cell wall adhesins. Among the (putative) adhesin-type proteins, the Epa proteins stand out, which represent a subfamily of lectin-type proteins. The biological functions of most of the wall adhesins are not known, except for a very limited number of adhesins of the Epa subfamily. A deeper understanding of the fundamental molecular mechanisms that regulate the activity of adhesins and their role in the pathogenesis of the infection is crucial to develop new strategies that allow the prevention and treatment of these candidiasis.
Fungal wall adhesins are modular proteins (Fig. 1); their precursors contain signal peptides to enter the ER and GPI anchors, and the mature proteins comprise an N-terminal domain, which generally determines the ligand-binding specificity of adhesins, followed by a low-complexity domain that in most cases contains internal tandem repeats. These internal repeats play an important role in the adhesiveness of the fungus and in the exposure of the ligand-binding domains modulated by the number of repeat copies and, consequently, by the variations in size of the genes encoding adhesins in clinical isolates.
Our data show that there are large phenotypic variations between clinical isolates of C. glabrata in its ability to adhere to abiotic surfaces of medical importance. We hypothesize that these more adherent (hyperadherent) strains are better colonizers of host tissues than the reference strains and that these hyperadherent phenotypes are governed by cell wall adhesins not yet characterized. Therefore, the main objective of our work is to identify and functionally characterize the adhesins that govern the hyperadherent phenotypes of clinical isolates. For the identification of new adhesins we use established protocols for the identification of cell wall proteins by mass spectrometry. With some of the most promising candidates, we carry out more detailed characterization studies by cloning and heterologous expression, and studies that define their ligand binding properties. We study, in vivo e in vitro, its relevance in the virulence of Candida using mutants with deletions, by phenotypic assays. We also plan to study the activity of different antifungal agents on adhesion capacity, biofilm formation, and experimental virulence of hyperadherent clinical isolates and specific adhesin deletion mutants.
These studies on adhesins are carried out in collaboration with the groups of Prof. G. Quindós (University of the Basque Country, Bilbao), Drs. M. Weig and O. Bader (Göttingen University, Germany) and Dr. N. Chauhan (Rutgers University, New Jersey, USA). Through other studies, the Medical Mycology group also collaborates with groups at the University of Valencia (Prof. E. Valentin), the University of Amsterdam, the Netherlands (Prof. C. de Koster), and the University of La Serena, Chile. (Prof. L. Castillo), among others.
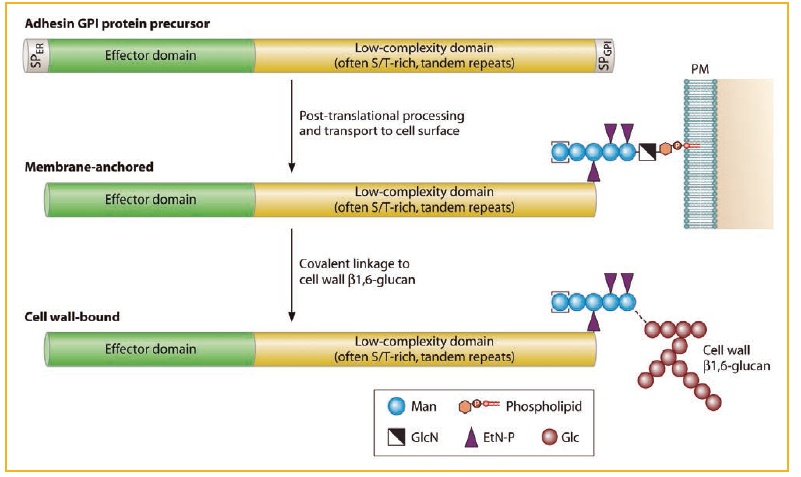
Figure 1. Generic structure and post-translational processing, leading to the incorporation of adhesins into the fungal cell wall. N- and O-glycosylation of proteins is not shown for simplicity.
Representative bibliography
Gioti A, et al., De Groot PWJ, Butler G, Heitman J, Scheynius A. (2013). Genomic Insights into the Atopic Eczema-Associated Skin Commensal Yeast Malassezia sympodialis. MBio 4: e00572-00512.
De Groot PWJ, Martinez AI, Castillo L. 2013. A genomic inventory of cell wall biosynthesis in the ubiquitous plant pathogen Botrytis cinerea. In Mora Montes HM (ed.), The Fungal Cell Wall, vol. In press. Nova Science Publishers, Inc., Hauppauge (NY).
De Groot PWJ, Bader O, De Boer AD, Weig M, Chauhan N. (2013). Adhesins in human fungal pathogens: glue with plenty of stick. Eukaryot. Cell 12: 470-481.
Wagener J , Weindl G , De Groot PWJ , De Boer AD , Kaesler S , Thavaraj S , Bader O , Mailänder-Sanchez D , Borelli C , Weig M , Biedermann T , Naglik JR , Korting HC , Schaller M . (2012). Glycosylation of Candida albicans cell wall proteins is critical for induction of innate immune responses and apoptosis of epithelial cells. PLoS ONE 7: e50518.
TheVISS K, De Mello Tavares P, Xu D, Blankenship J, Vandenbosch D, Idkowiak-Baldys J, Govaert G, Bink A, Rozental S, De Groot PWJ, Davis TR, Kumamoto CA, Vargas G, Nimrichter L, Coenye T, Mitchell A, Roemer T, Hannun YA, Cammue BPA. (2012). The plant defensin RsAFP2 induces cell wall stress, septin mislocalization and accumulation of ceramides in Candida albicans. Mol. Microbiol. 84: 166-180.
Gelis S, De Groot PWJ, Castillo L, Moragues MD, Sentandreu R, Gómez MM, Valentín E. (2012). Pga13 in Candida albicans is localized in the cell wall and influences cell surface properties, morphogenesis and virulence. Fungal Genet. Biol. 49: 322-331.
De Groot PWJ, Brandt BW. (2012). ProFASTA: A pipeline web server for fungal protein scanning with integration of cell surface prediction software. Fungal Genet. Biol. 49: 173-179.
Bader O, Schwarz A, Kraneveld EA, Tangwattanchuleeporn M, Schmidt P, Jacobsen MD, Groß U, De Groot PWJ, Weig M. (2012). Gross karyotypic and phenotypic alterations among different progenies of the Candida glabrata CBS138/ATCC2001 reference strain. PLoS ONE 7: e52218.
Laforet L, Moreno I, Sánchez-Fresneda R, Martínez-Esparza M, Martínez JP, Argüelles J-C, De Groot PWJ, Valentín E. (2011). Pga26 mediates filamentation and biofilm formation and is required for virulence in Candida albicans. FEMS Yeast Res. 11: 389–397.
Kraneveld EA, De Soet JJ, Deng DM, Dekker HL, De Koster CG, Klis FM, Crielaard W, De Groot PWJ. (2011). Identification and differential gene expression of adhesin-like wall proteins in Candida glabrata biofilms. Mycopathologia 172: 415-427.
De Boer AD, De Groot PWJ, Weindl G, Schaller M, Riedel D, Diez-Orejas R, Klis FM, De Koster CG, Dekker HL, Groß U, Bader O, Weig M. (2019). A Live Candida albicans cell wall protein Rhd3/Pga29 is abundant in the yeast form and contributes to virulence. Yeast 27: 61-624.
De Groot PWJ, Kraneveld EA, Yin QY, Dekker HL, Groß U, Crielaard W, De Koster CG, Klis FM, Weig M. (2008). The cell wall of the human pathogen Candida glabrata: differential incorporation of novel adhesin-like wall proteins. Euk. Cell 7: 1951-1964.
De Groot PWJ, Klis FM. (2008). The conserved PA14 domain of cell wall-associated fungal adhesins governs their glycan binding specificity. Mol. Microbiol.68: 535-537.
