
Photo of GrYou are there. From left to right: Iván Butrón Ollo, Diego Vizcaíno Sáez, José Muñoz Dorado, Silvia María Conde Sánchez, Gisela García Ruiz, Juana Pérez Torres, Andrea Pradillo López, Francisco Javier Marcos Torres, Aurelio Moraleda Muñoz
The BIO-318 procariotic development group is part of the Department of Microbiology of the University of Granada (website: https://t.co/CnNJBRNFqa; Account in X: https://x.com/MyxoUGR; Instagram account: https://www.instagram.com/myxo_ugr/). This group is led by Dr. José Muñoz-Dorado and is currently trained by two university professors (José Muñoz Dorado and Juana Pérez Torres), a university professor (Aurelio Moraleda Muñoz), a permanent work teacher (Francisco Javier Marcos Torres) and different master's and degree students.
The group, distinguished with the “Universidad de Granada Award for Research of Excellence” in 2011, conducts investigations with the Model Mixobacteria Myxococcus xanthus, which presents unique social and multicellular behavior among prokaryotes. In our studies we combine classic techniques of microbiology, molecular biology and microscopy, with massive, transcriptomic, bioinformatic and genomic sequencing technologies.
Mixobacteria: prokaryotic signaling and multicellularity model
Mixobacteria (phylum Myxococcota), in general, and M. xanthus, In particular, they are microbial predators of the soil that have a peculiar multicellular cell cycle. Thus, in the presence of nutrients, the cells move by sliding in a coordinated way forming swarms. When swarms come into contact with the dam, mixobacteria penetrate the colony and produce the lysis of their prey through various mechanisms, including the release of hydrolytic enzymes and secondary metabolites. However, under conditions of nutrient shortages, mixobacteria, moving coordinated and collectively, begin a development program and exchange extracellular chemical signals, as well as signs dependent on physical contact, to form aggregates and build fruitful bodies of macroscopic size. During the development cycle there are three possible cell destinations: i) the cells located within the fruitful bodies differ in spherical mixesports, which are resistant to several adverse conditions; i) surrounding the fruitful body appears a monolayer of peripheral bacilli, not differentiated and that remain in a persistent state; and iii) a majority portion of swarm cells experiences autolysis by programmed cell death. Mixómpores ensure survival during starvation or desiccation, and are capable of dispersing other environments and germinating when nutritional conditions improve (Figure 1a). Both depredation and development cycle are processes that require the coordinated action of a multitude of cells, highlighting the richness of signaling systems present in these bacteria to quickly perceive and communicate the changes in the environment and coordinate a group response.
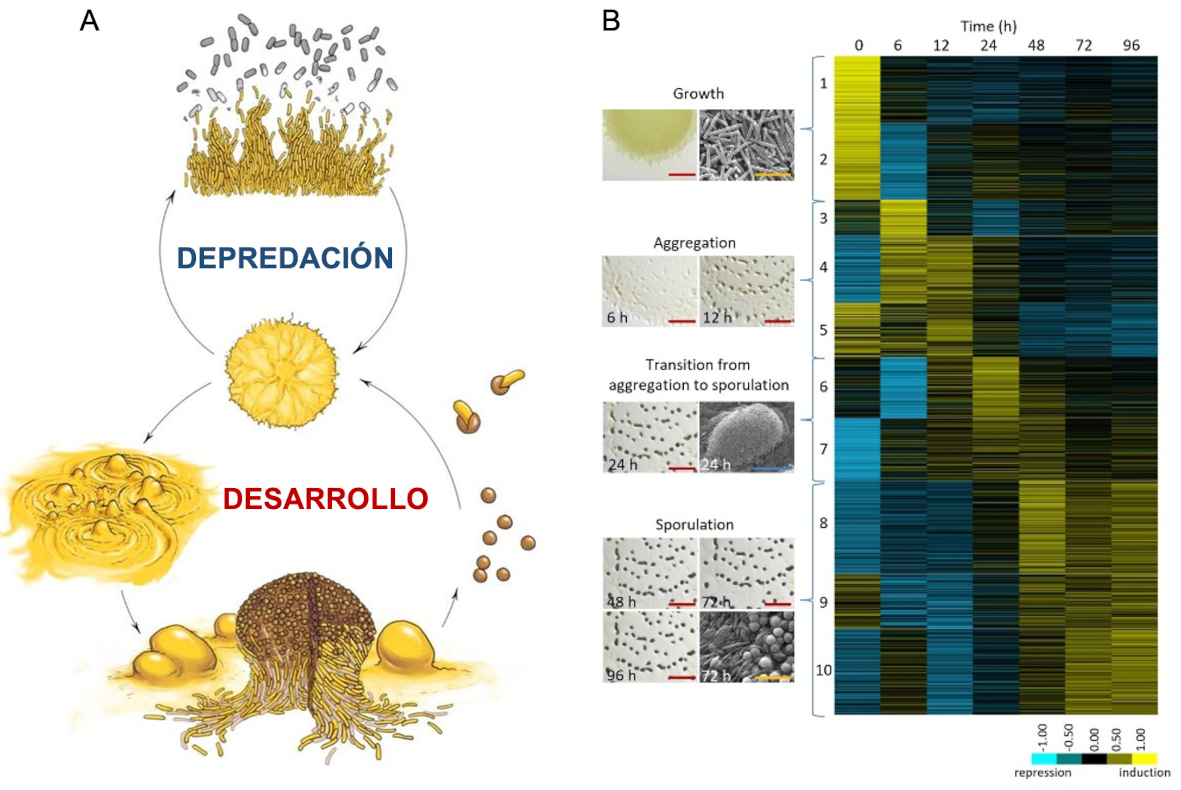
Figure 1. Multicellularity and signaling in M. Xanthus. A. M. Xanthus's life cycle highlighting its vegetative growth phase where the cooperative predation of other microorganisms and the development cycle where fruitful bodies with mixósporas resistant to adverse conditions will take place. B. Transcriptomic changes during the development cycle of M. Xanthus.
Our interest is to unravel the molecular processes that govern the complex social behaviors of M. xanthus To optimize its use of resources and their survival by adopting a multicellular lifestyle, analogously to eukaryotic organisms. In this sense, our studies on the life cycle of M. xanthus They have allowed deciphering the dynamics of transcriptoma throughout the development cycle (Figure 1b) and predation (Figure 2a). We are also interested in the response of this bacterium to various stimuli of its environment. At present, our two main research pathways focus on identifying and characterizing the molecular mechanisms that act during bacterial predation and during the adaptation of this bacterium to environmental changes.
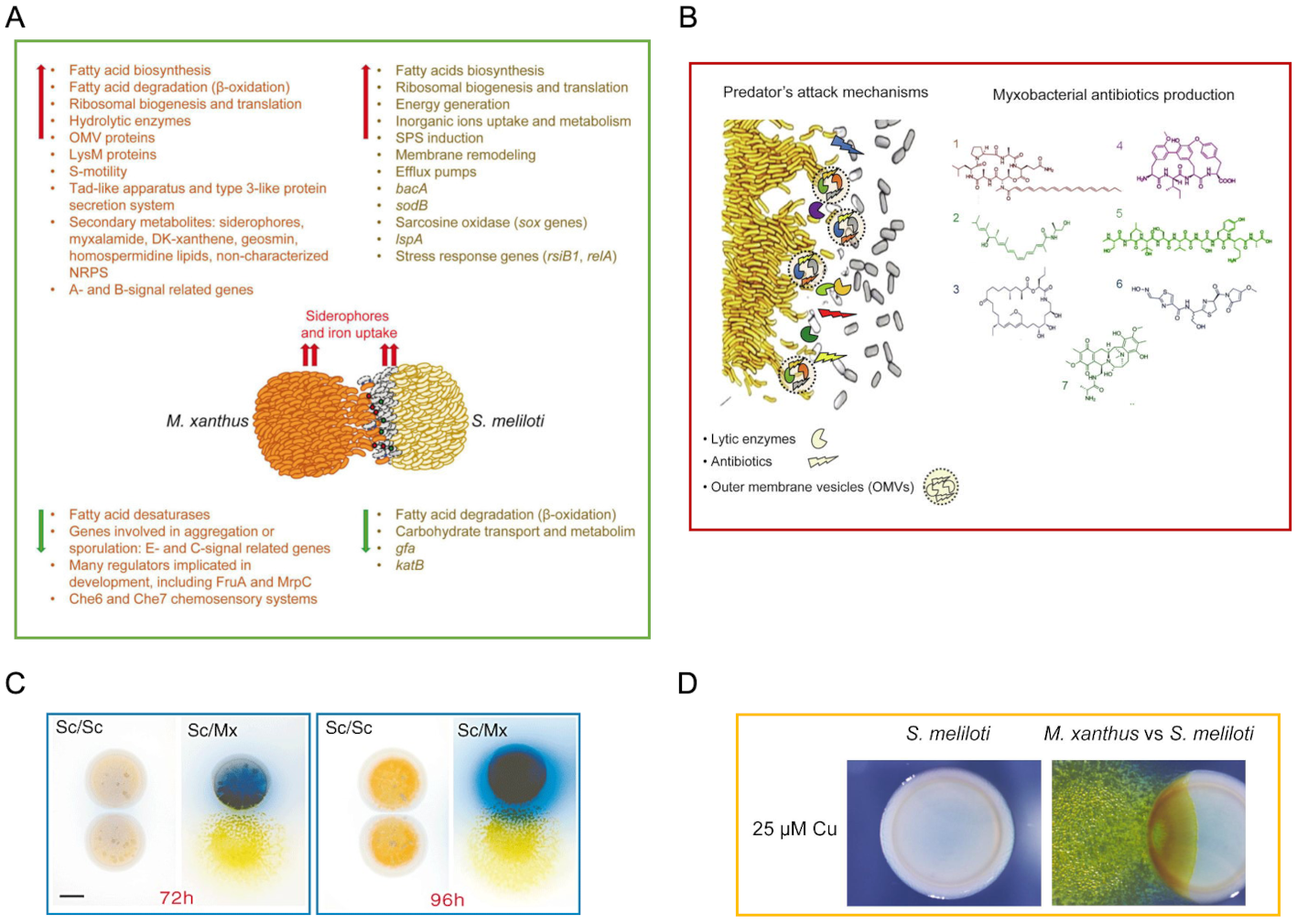
Figure 2. Bacterial predation in M. Xanthus. A. Analysis of the Predatosoma of M. Xanthus and the Defensoma of S. Meliloti during its interaction. B. Production of antibiotics by M. Xanthus during predation. C. Actinorrodin production by S. coelicolor when it is predated by M. Xanthus. D. melanin production by S. Meliloti when it is predated by M. Xanthus in the presence of copper.
Bacterial predation: basic studies and ecological, evolutionary, biotechnological and agricultural consequences
Bacterial predation has been proposed as a driving force of evolution. In addition, the structure and composition of predatory bacterial communities begin to recognize themselves as an important factor in biodiversity, due to their potential role in the control and modeling of populations of other microorganisms in the environment.
- xanthus It is able to prey a wide variety of positive and negative gram bacteria, as well as fungi. Our group studies the depredation strategies of M. xanthus on other relevant soil bacteria, such as Streptomyces coelicolor O Sinorhizobium meliloti. In relation to this, we have determined pretosoma (genes of M. xanthus involved in predation) and defensoma (prey genes involved in defense) during interaction M. xanthus – S. meliloti through mass sequencing techniques (RNA-SEQ), genomics, and classical techniques of molecular biology and microbiology (Figure 2a). This research has shed light on the processes used by these predators to recognize, kill and lisar their prey.
On the other hand, since mixobacteria have genetic potential to produce a wide battery of antibiotics and other secondary metabolites (Figure 2b), studies in vivo In front of dams they can contribute to the discovery of new products with biological activity and could open new horizons in its application to biocontrol processes against pathogenic bacteria, as an alternative to antibiotics.
In addition, the study of dam defense mechanisms is allowing us to reveal new bacterial strategies against depredation, such as the induction of the production of new antibiotics in the case of S. coelicolor (Figure 2c) or protection metabolites against oxidative stress, as in the case of Sinorhizobium, that produces melanin to protect yourself from M. xanthus When it is predicted in the presence of copper (Figure 2D).
Adaptation of M. xanthus to environmental changes
- xanthus You must complete your life cycle in the presence of natural compounds of your environment. One of these compounds is copper, which is found in concentrations that can range between 2 and 100 mg/kg of soil. This metal is essential for life, since it acts as a cofactor of numerous enzymes involved in vital processes but, nevertheless, if it is in excess, it is also extremely toxic, mainly due to the formation of reactive oxygen species. Due to this double effect, the cells have different mechanisms to ensure adequate copper intracellular levels. M. xanthus It offers us the opportunity to study the cellular response throughout a full life cycle, which has allowed us to elucidate the global response to copper in monocultures and in the presence of prey (Figure 3a). In addition, these studies have given us the possibility of identifying several components of the copper response that could be involved in the cross resistance to antibiotics. The study of the regulation of the genes involved in the homeostasis of copper has also allowed us to describe new regulation systems, including a new group of sigma ECF factors dependent on metals that, due to its ability to join metals and its mechanism of independent action of antisigm factors, has meant the creation of a new group of metallorregulators (Figure 3b).
In addition to copper, our group has also studied the effects on M. xanthus of other elements present in the environment such as light, cadmium, zinc or iron. About the latter, we have recently described that he plays a key role during predation influencing the result of this interaction (Figure 3c).
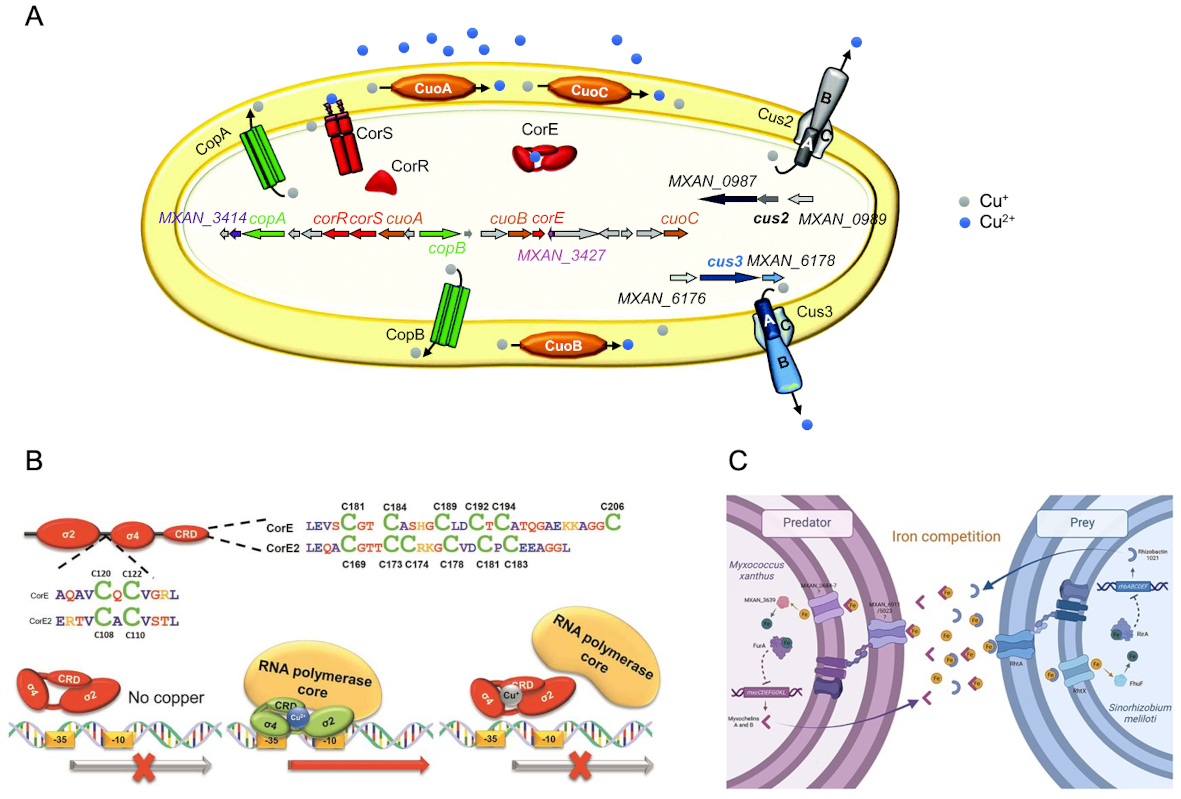
Figure 3. Adaptation to environmental changes in M. Xanthus. A. Global response to copper in M. Xanthus. B. Mechanism for regulating the SIGMA of the ECF Core-Like type. C. Iron paper during the bacterial predation of M. Xanthus.
Selected works (last 10 years)
Kroos, L., Wall, D., Islam, S.T., Whitworth, D.E., Muñoz-Dorado, J., Higg, P.I., Singer, M., Mauriello, E.M. , et al. (2025). Milestones in the development of Myxococcus xanthus as a model multicellular bacterium. Journal of Bacteriology, 207:e0007125
Contreras-Moreno, F.J., Moraleda-Muñoz. A., Marcos-Torres, F.J., Cuéllar, V., Soto, M.J., Pérez, J. and Muñoz-Dorado, J. (2024). Siderophores and competition for iron govern myxobacterial predation dynamics. ISME Journal, 2:wrae077.
Contreras-Moreno, F.J., Pérez, J., Muñoz-Dorado, J., Moraleda-Muñoz, A. and Marcos-Torres, F.J. (2024). Myxococcus xanthus predation: an updated overview. Frontiers in Microbiology, 15: 1339696
Soto, M.J., Pérez, J., Muñoz-Dorado, J., Contreras-Moreno, F.J., and Moraleda-Muñoz, A. (2023). Transcriptomic response of Sinorhizobium meliloti to the predatory attack of Myxococcus xanthus. Frontiers Microbiology 14:1213659.
Marcos-Torres, F.J., Moraleda-Muñoz, A., Contreras-Moreno, F.J., Muñoz-Dorado, J. and Pérez, J. (2022). Mechanisms of action of non-canonical ECF sigma factors. International Journal of Molecular Sciences, 23: 3601-3614
Pérez, J., Contreras-Moreno, F.J., Muñoz-Dorado, J., Moraleda-Muñoz, A. (2022). Development versus predation: Transcriptomic changes during the lifecycle of Myxococcus xanthus. Frontiers Microbiology 13: 1004476
Li, Y.P., Ben-Fakih, I., Chi-Fru, E., Moralda-Muñoz, A., Li, X., Rosen, B.P., Yoshinaga, M. y resing, C. (2021). Antimicrobial activity of metals and metalloids. Annual Review of Microbiology, 75:1, 175-197
Marcos-Torres, F.J., Volz, C., and Müller R. (2020). An ambruticin-sensing complex modulates Myxococcus xanthus development and mediates myxobacterial interspecies communication. Nature Communications, 11: 5563.
Contreras-Moreno, F.J., Muñoz-Dorado, J., García-Tomsig, N.I., Martínez-Navajas, G., Pérez, J., and Moraleda-Muñoz. (2020). Copper and melanin play a role in Myxococcus xanthus predation on Sinorhizobium meliloti. Frontiers in Microbiology, 11: 94.
Pérez, J., Contreras-Moreno, F.J., Marcos-Torres, F.J., Moraleda-Muñoz, A. and Muñoz-Dorado. J. (2020). The antibiotic crisis: How bacterial predators can help. Computational and Structural Biotechnology Journal, 118: 2547–2555.
Muñoz-Dorado J., Moraleda-Muñoz, A., Marcos-Torres, F.J., Contreras-Moreno, F.J., Martin-Cuadrado, A.B., Schrader, J.M., Higgs, P.I., and Pérez, J. (2019). Transcriptome dynamics of the Myxococcus xanthus multicelular developmental program. eLife: e50374.
Moraleda-Muñoz, A., Marcos-Torres, F.J., Pérez, J., and Muñoz-Dorado J. (2019). Metal-responsive RNA polymerase extracytoplasmic function (EFC) sigma factors. Molecular Microbiology 112: 385-398.
Pérez, J., Muñoz-Dorado J., and Moraleda-Muñoz, A. (2018). The complex global response to copper in the multicellular bacterium Myxococcus xanthus. Metallomics. 10: 876-886.
Muñoz-Dorado J., Marcos-Torres, F.J., Moraleda-Muñoz, A., and Pérez J. (2016). Myxobacteria: Moving, killing, feeding, and surviving together. Frontiers in Microbiology. 4: 1-18.
Marcos-Torres, F.J., Pérez, J., Gómez-Santos, N., Moraleda-Muñoz, A., and Muñoz-Dorado, J. (2016). In depth analysis of the mechanism of action of metal-dependent sigma factors: characterization of CorE2 from Myxococcus xanthus. Nucleis Acids Research. 44: 5571-5584.
Pérez, J., Moraleda-Muñoz, A., Marcos-Torres, F.J., and Muñoz-Dorado, J. (2016). Bacterial predation: 75 years and counting!. Environmental Microbiology. 18: 766-779.
