
Group photo. From left to right, Yasser Mahmmod, Miguel Blanco, Pau Obregón, Virginia Aragón, Berenice Plasencia, Flor Correa, Carlos Neila, Sergi López, Marina Sibila, Nuria Galofré, Eva Huerta and Mar Costa
There is great pressure to reduce the use of antimicrobials, due to the constant increase in resistance and the imminent problem for the health of animals and people. In Spain, work is being done through a national plan, the PRAN, and, although the use of antimicrobials is still high, it is worth noting the drastic reduction of 97% in the use of colistin in pigs between 2015 and 2018. In pig production , respiratory diseases represent one of the biggest health problems and consequently are one of the main causes of antimicrobial use in this sector.
One of the respiratory pathogens of importance in the pig sector is Haemophilus parasuis, which causes Glässer's disease (fibrinous polyserositis, including meningitis and arthritis). For years our group has focused on studying the pathogenicity of this bacterium with the ultimate goal of developing an effective vaccine. Through genetic typing studies, virulence assays and experimental infections in piglets, we defined different degrees of virulence in field strains and detected a group of molecules associated with said virulence, the trimeric VtaA autotransporters. VtaAs are antigenic proteins located on the outer membrane, which are expressed during pig infection. VtaAs are virulence factors, involved in mechanisms such as adhesion to extracellular matrix proteins or resistance to phagocytosis. The analysis of its structure allowed us to define an epitope exposed on the bacterial surface, common to all the VtaA of the virulent strains. Antibodies against this epitope are opsonizing and would allow directing the response specifically to virulent strains. In addition, since the epitope is present in all VtaAs associated with virulence, antigenic exchange would be avoided (“antigenic switching”) between different VtaAs.
In recent years our interest has expanded to other porcine pathogens, such as Mycoplasma hyorhinis and Streptococcus suis, and the role of the nasal microbiota. The nasal cavity is the natural entry route for respiratory pathogens, and there they encounter the first barrier against infection, the nasal microbiota. Comparing this microbiota in piglets from farms with Glässer's disease versus that of healthy piglets allowed us to determine that the nasal microbiota can predispose piglets to disease. We note that several bacterial taxa, such as Bacteroidales, Lactobacillales and Clostridiales, were reduced in the nasal microbiota in weaned piglets that later developed Glässer's disease. Glässer's disease is caused by virulent strains of H.parasuis, but this bacterium also has non-virulent strains, which can protect against the disease. The microbiota associated with these two types of strains of H parasuis turned out to be different, detecting some bacterial families that could facilitate the colonization of virulent strains of H.parasuis (and therefore increase the risk of disease), and others that could facilitate the colonization of non-virulent strains (and therefore promote protection against Glässer's disease). These findings support the idea that specific interventions can be designed to achieve an adequate microbiota composition to ensure piglet protection. But the role of the nasal microbiota is not specific to Glässer's disease, and piglets that later developed polyserositis due to M. hyorhinis they had a very different composition to that of piglets from healthy farms, with different bacterial genera associated with health and disease.
Additionally, antimicrobials can have side effects on the beneficial microbiota. We have recently shown that the elimination of perinatal antimicrobial treatments produces an increase in bacterial diversity in the nasal microbiota. This increase in diversity has long-term beneficial effects, with a reduction in mortality and medication costs, and an increase in productivity. The most significant changes in the composition of the microbiota upon removal of antimicrobials were an increase in Prevotella and Lactobacillus, and a reduction of Moraxella and Bergeyella (Figure 1).
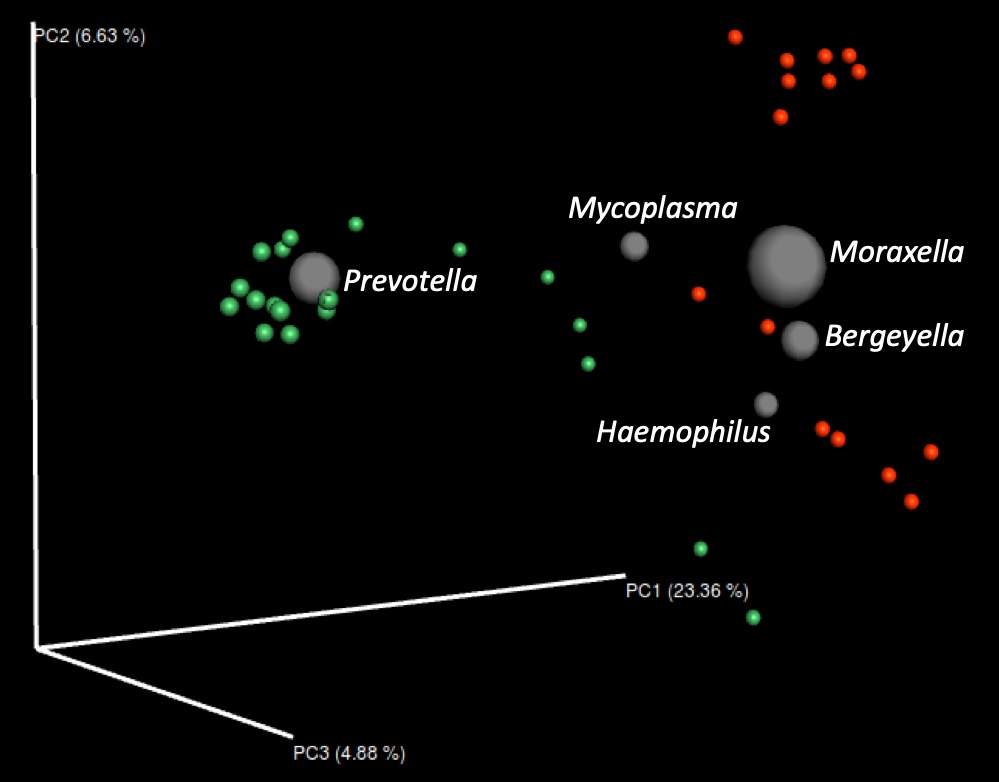
Figure 1. Principal coordinates (PC) analysis showing the beta diversity of the nasal microbiota of weaning piglets (3-4 weeks of age) before (red dots) and after (green dots) the removal of perinatal antimicrobial treatments. The gray spheres indicate the 5 most abundant genera and are proportional to their relative abundance.
Our group also has experience in other respiratory pathogens of great economic importance worldwide, such as Mycoplasma hyopneumoniae and Actinobacillus pleuropneumoniae, causal agents of pneumonia and pleuropneumonia in pigs. It is worth highlighting the fine-tuning of pig disease models, which are very useful for studies of new therapeutic products, vaccines or for the validation of diagnostic tools. In addition, it has been determined that the diagnosis of M. hyopneumoniae improvement using laryngeal swabs in live animals and lower respiratory tract samples (brochoalveolar lavage or lung swab) in dead animals, and methods of quantifying lung lesions have been established for both pathogens.
Our future objective will continue to be the control of swine respiratory diseases in the absence of antibiotics, with special emphasis on alternative strategies, such as the development of probiotics.
Representative Publications
Betlach AM, Maes D, Garza-Moreno L, Tamiozzo P, Sibila M, Haesebrouck F, Segalés J, Pieters M. (2019). Mycoplasma hyopneumoniae variability: Current trends and proposed terminology for genomic classification. Transbound Emerg Dis 66:1840-54.
Costa-Hurtado M, Garcia-Rodriguez L, Lopez-Serrano S and Aragon V. Abushattal S, Vences A, Dos Santos NMS, Do Vale A and Osorio CR. Haemophilus parasuis VtaA2 is involved in adhesion to extracellular proteins. Vet Res 50:69.
Correa-Fiz F, Gonçalves Dos Santos JM, Illas F and Aragon V. (2019). Antimicrobial removal on piglets promotes health and higher bacterial diversity in the nasal microbiota. Sci Rep 9:6545.
Garza-Moreno L, Segalés J, Aragón V, Correa-Fiz F, Pieters M, Carmona M, Krejci R and Sibila M. (2019). Characterization of Mycoplasma hyopneumoniae strains in vaccinated and non-vaccinated pigs from Spanish slaughterhouses. Vet Microbiol 231:18-23.
Garcia-Morante B, Dors A, León-Kempis R, Pérez de Rozas A, Segalés J, Sibila M. (2018) Assessment of the in vitro growing dynamics and kinetics of the non-pathogenic J and pathogenic 11 and 232 Mycoplasma hyopneumoniae strains. Vet Res 49:45.
Fernández-Aguilar X, Gottschalk M, Aragon V, Càmara J, Ardanuy C, Velarde R, Galofré-Milà N, Castillo-Contreras R, López-Olvera JR, Mentaberre G, Colom-Cadena A, Lavín S and Cabezón O. (2018). Urban Wild Boars and Risk for Zoonotic Streptococcus suis, Spain. Emerg Infect Dis 24(6):1083-6.
Mathieu-Denoncourt A, Letendre C, Auger JP, Segura M, Aragon V, Lacouture S y Gottschalk M. (2018). Limited interactions between Streptococcus suis and Haemophilus parasuis in in vitro co-infection studies. Pathogens 7(1). pii: E7.
Howell KJ, Weinert LA, Peters SE, Wang J, Hernandez-Garcia J, Chaudhuri RR, Luan SL, Angen Ø, Aragon V, Williamson SM, Langford PR, Rycroft AN, Wren BW, Maskell DJ y Tucker AW. (2017). “Pathotyping” Multiplex PCR Assay for Haemophilus parasuis: a Tool for Prediction of Virulence. J Clin Microbiol 55:2617-28.
Correa-Fiz F, Galofre-Mila N, Costa-Hurtado M and Aragon V. (2017). Identification of a surface epitope specific of virulent strains of Haemophilus parasuis. Vet Microbiol 198:116-20.
Correa-Fiz F, Fraile L and Aragon V. (2016). Piglet nasal microbiota at weaning may influence the development of Glässer’s disease during the rearing period. BMC Genomics 17:404.
